Week Four: Snake oil, EU venture takes a hit, and Uber's new shuttles
Does red light therapy actually work? And did Uber just decide to make a bigger Uber Pool??
Housekeeping
Welcome to the fourth edition of Venture Vantage. We’ll be exploring topics related to tech and the venture ecosystem.
Apologies for getting this edition out so late—it’s been a busy week, to say the least.
There will be some slight modifications to the format, adding a short “white hot” section that names and links topics that are, in my opinion, interesting areas for investment.
As always, please hit me back with feedback and comments—I’m constantly seeking ways to make this newsletter a more valuable read.
Diving right in and keeping things brief:
The Meat & Potatoes
Is red light therapy fairy dust?
Context:
I recently came across Solawave. The device uses “Red, Deep Red, Near-Infrared, and Amber Light Therapies to deliver a complete, pain-free treatment in just 3 minutes.”
They claim it helps with “aging in the delicate eye area, including fine lines, wrinkles, crow’s feet,” and more. Solawave’s “FDA Cleared” claim stuck out to me.
My mom recently hurt herself, and she bought a PlatinumLED Biomax 900 to help with recovery. PlatinumLED has very similar claims, and cites that they are a FDA Class II Medical Device.
Red light therapy research began with NASA studying how it can help plants grow in space and how it could potentially help astronauts heal faster.
Supposedly, red light therapy works by boosting the strength of mitochondria, which we all know from 7th-grade science is the powerhouse of the cell. When the mitochondria are stimulated and strengthened, they produce more ATP, which is the main energy source of cells. This, in turn, allows the cells to function more effectively and repair damage more quickly.
All this has made me think: does red light therapy actually work, or is it snake oil?
Vantage:
There are a few randomized controlled trials to show that red light therapy is effective for various use cases, including:
Skin rejuvenation: red light therapy (RLT) worked to stimulate collagen production, leading to a reduction in wrinkles and improved skin smoothness.
Hair growth: randomized controlled trials have found red light therapy to be effective in treating androgenetic alopecia (hereditary hair loss). These trials show that red light therapy can increase hair density and thickness.
Pain and inflammation: A 2020 study showed that RLT could improve tissue repair and reduce joint pain. RLT was effective in reducing inflammation, especially in patients with musculoskeletal conditions and osteoarthritis.
The biggest criticism of the above trials is that they were not nearly large enough sample sizes or long enough in duration to show efficacy and to show that the effects are long-lasting.
Some of these RLT devices are FDA-cleared. Now, what does that mean?
It does not necessarily mean that the device is proven to be highly effective. Instead, it means that the device has met specific safety standards set by the FDA and is considered low risk for public use. Meaning that it doesn’t need to be effective to be FDA-cleared; it just needs to be safe.
Many red light therapy devices are cleared under what’s known as the 510(k) pathway, which requires the manufacturer to demonstrate that the device is “substantially equivalent” to another device already on the market. This primarily assesses safety, not necessarily effectiveness.
The terms FDA-cleared and FDA-approved do not mean the same thing. The phrase “FDA-approved” is used for more stringent review processes typically reserved for drugs and high-risk medical devices. It means the device has undergone clinical testing to demonstrate both safety and efficacy. However, most red light therapy devices are not FDA-approved in this sense—they are FDA-cleared, meaning they are cleared as safe for general use.
In short, FDA-cleared usually refers to Class I and Class II devices, cleared through a 510(k) pathway, whereas Class III devices are much higher risk and are FDA-approved through a premarket approval (PMA).
Class I and Class II are low to moderate risk devices, usually like RLT devices, blood pressure monitors, or wheelchairs. Class III medical devices often support or sustain life or have the potential for high risks, like pacemakers, heart valves, or implants.
In conclusion, RLT studies are preliminarily effective and, while they are safe, have not been robustly proven to be effective.
This opens the market to opportunities for far more effective non-invasive therapies. Technologies that use EMFs/EMPs to neuromodulate parts of the brain have been proven to be far more effective for symptoms like pain, and companies that leverage these technologies have a higher potential for success in my eyes.
News, Deals, and Pretty Things
Ultra-low funding levels for the EU
Context:
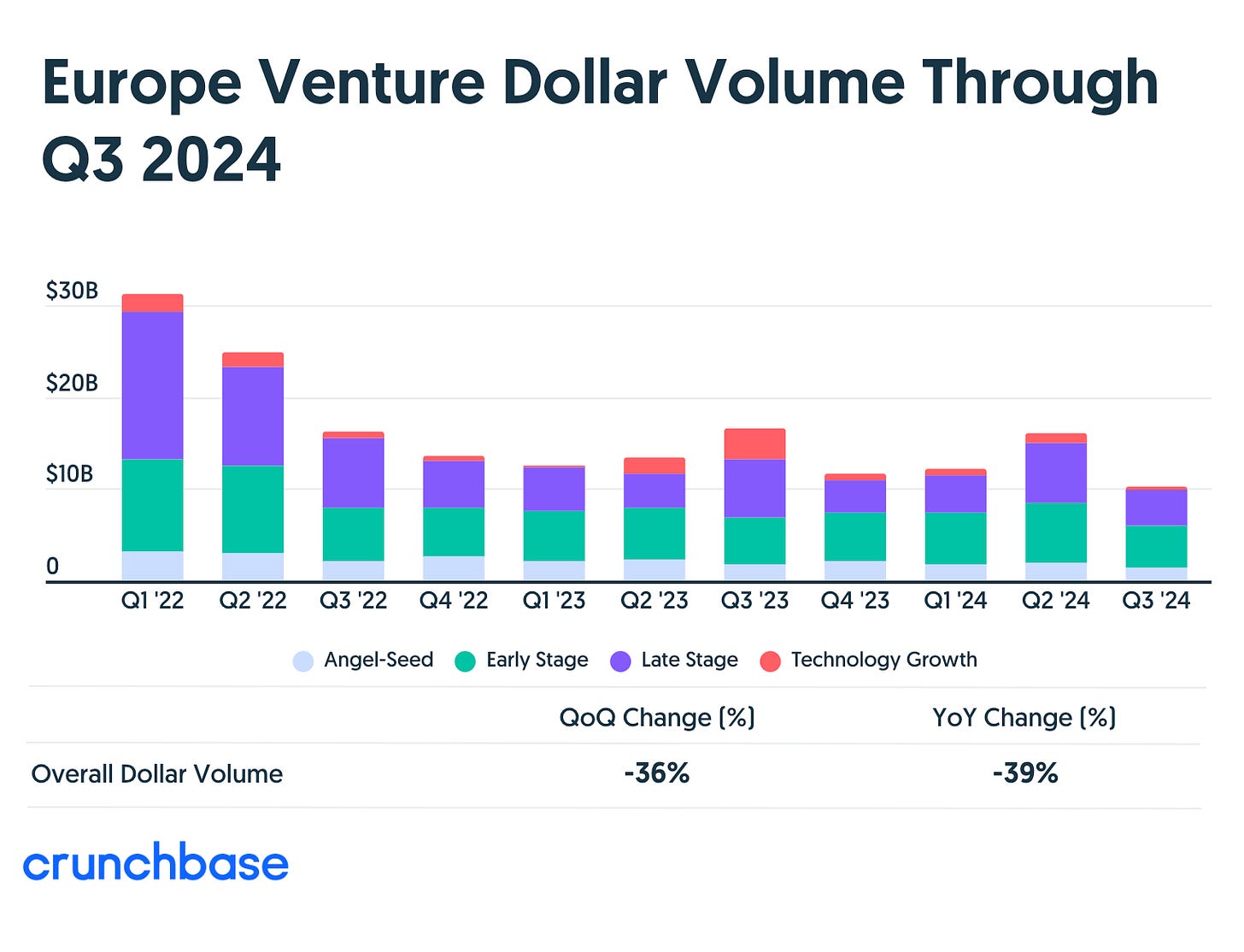
Crunchbase is reporting that venture funding numbers in Europe have hit their lowest quarter in the last four years.
European startups raised $10b in Q3’24. That’s 1/3 of what they raised in Q1’22.
It appears that late-stage and growth rounds have taken the biggest hit.
Vantage:
This likely means that there are better deals available in the EU. As companies struggle to raise funds, valuations will drop. Supply and demand!
This might mean more European-based startups relocating to the US (or just incorporating in the US) with the hopes of securing some more free-flowing venture funding. This could potentially disrupt US markets by increasing competition here in the States.
This could forecast trouble for the European IPO market. With fewer startups raising now, there will be fewer startups to go public in future years. The decline of funding across all stages could mean a multi-year IPO decline.
Nvidia comes out swinging
Context:
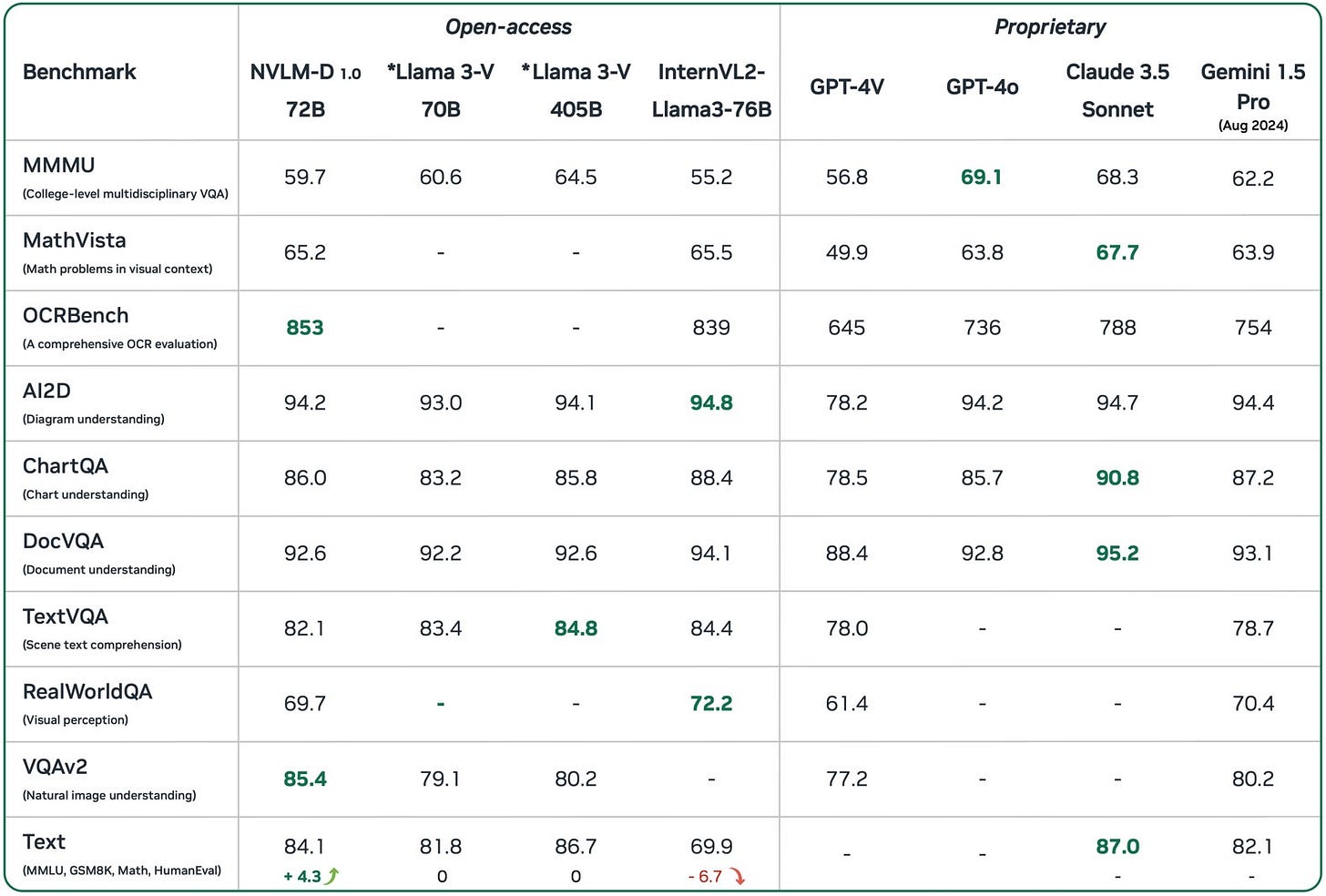
Nvidia semi-quietly released NVLM 1.0, their own large multimodal language model that performs at least as well as GPT-4o.
The model performs exceptionally well in a number of areas, including image recognition, description, and analysis.
From my understanding, this new NVLM overtook Meta’s Llama series as the best-performing open source LLM.
Vantage:
A big question I have is, how much less power does NVLM use than other comparable LLMs? Something we often see is that software developed by the hardware manufacturer tends to run more smoothly on that hardware. This isn’t a hard and fast rule but more an anecdotal observation. Does NVLM run more efficiently on Nvidia’s GPUs?
I wonder how much this threatens OpenAI’s stability in the market. With the emergence of really strong open-access LLMs, does that allow people to add their own UI and build a really hyper-specialized LLM that can outperform GPT-4o’s “My GPTs” feature? Maybe I don’t have as comprehensive of an understanding of this as I should.
Uber’s new shuttles
Context:
Uber announced today that they’re launching a new shuttle service in NYC.
It’ll offer rides to NYC’s airports for less than $20—a significant decline from the standard $60-120 that an Uber trip usually costs.
Up to 18 passengers can ride in each Uber shuttle, making Uber a lot more money for nearly the same variable cost an independent driver would incur for taking 1-4 people that an UberX would normally carry.
Vantage:
If the pilot program for Uber shuttles goes well in New York City, it has the potential to expand into other cities. This could mean trouble for local shuttle services, like SuperShuttle.
Uber could potentially be double dipping on ride volume stats with this new service, as some customers would take a regular Uber ride to the shuttle pickup point. It could drop total revenue from that customer for a trip to the airport, but it would count for two rides in that trip rather than just one. Although, I don’t think it’ll be significant enough to drastically change Uber’s rider stats.
This is likely in response to Uber Share (previously Uber Pool) not being a reliable or predictable way to get to the airport on time. Uber can capture the revenue of shared rides while offering a tighter schedule for time reliability.
White Hot
Human Health:
Menopause care, helping women find effective solutions to alleviate menopause symptoms.
Yamanaka factors.
Wearables that monitor unique data points (noninvasive glucose, blood pressure, hydration, sweat loss, etc).
Future of Work:
Customer service tools—think Superhuman but for enterprise customer service teams.
CRMs for niche industries (think Affinity, but for other industries).
Software that allows business owners to rapidly create custom software.
Infrastructure:
Nano-satellite connectivity to replace legacy cellular carriers.
Automotive tires that never have to be replaced (a $61B industry in 2022 in the US).
Micro-nuclear power solutions (best example I can think of is Aalo).
Some cool stuff on my radar
Landscape is a super cool sourcing tool for venture funds that I saw a demo of today. It has a native Affinity integration, and has the potential to be so powerful.
The Oura Ring 4 also came out this past week. It boasts a better battery life, a full titanium design, marginally more accurate sensors, etc. It seems like a lot of the new generations of devices that came out this year, like the iPhone 16 series, have been iterative improvements on past generations rather than incorporating groundbreaking innovations. I’ve said it before and will say it again, I think the wearables space is ripe for disruption with some new data inputs.
I have come to the conclusion that Buck Mason’s Pima Tees have changed my life. I will wear nothing else ever again.
I’ve heard John Elliott is a close second, but I haven’t yet verified this info.
These Elwood hoodies are also epic, since we’re talking about clothes.
I haven’t yet received mine, but I think the Journeyman Pen by Wingback will be a solid new desk pen for me.
Closing
Thanks for taking time out of your Wednesday to read. Chag Sameach, wishing you an easy & meaningful fast.
As always, you can find me on X and LinkedIn, and I’d love to hear from you via email. Whether it’s talking startups or just shooting the shit, I’m always happy to connect.
Onto the next!
//Eli